使用者:DoroWolf/沙盒
| 這是DoroWolf的用戶沙盒。使用者沙盒是使用者頁面的子頁面,屬於使用者的測試區,不是維基百科條目。 公用沙盒:主沙盒 | 使用指南沙盒一、二 | 模板沙盒 | 更多…… 此用戶沙盒的子頁面: 外觀選項: 用字選項: 如果您已經完成草稿,可以請求志願者協助將其移動到條目空間。 |
| DoroWolf/沙盒 | |
|---|---|
| |
| |

| |
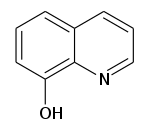
| IUPAC名 Quinolin-8-ol | |
| 別名 | 1-Azanaphthalene-8-ol, Fennosan H 30, Hydroxybenzopyridine, Oxybenzopyridine, Oxychinolin, Oxyquinoline, Phenopyridine, Quinophenol, Oxine, 8-Quinolinol |
| 識別 | |
| CAS號 | 148-24-3 |
| PubChem | 1923 |
| ChemSpider | 1847 |
| SMILES |
|
| InChI |
|
| InChIKey | MCJGNVYPOGVAJF-UHFFFAOYAG |
| ChEBI | 48981 |
| KEGG | D05321 |
| 性質 | |
| 化學式 | C9H7NO |
| 摩爾質量 | 145.16 g/mol g·mol⁻¹ |
| 外觀 | White crystalline needles |
| 密度 | 1.034 g/cm3 |
| 熔點 | 76 °C(349 K) |
| 沸點 | 276 °C(549 K) |
| 藥理學 | |
| ATC代碼 | G01AC30(G01),A01 D08 R02 |
| 危險性 | |
GHS危險性符號   
| |
| GHS提示詞 | Danger |
| H-術語 | <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="危險說明述有誤">HH301, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="危險說明述有誤">HH317, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="危險說明述有誤">HH318, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="危險說明述有誤">HH360D, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="危險說明述有誤">HH410 |
| P-術語 | <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP202, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP273, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP280, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP301 + P310, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP302 + P352, <span class="abbr skin-invert" style="color: blue; border-bottom: 1px dotted blue" title="防範說明有誤">PP305 + P351 + P338 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
8-Hydroxyquinoline (also known as oxine) is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which is used for the quantitative determination of metal ions.
In aqueous solution 8-hydroxyquinoline has a pKa value of ca. 9.9[1] It reacts with metal ions, losing the proton and forming 8-hydroxyquinolinato-chelate complexes.

The aluminium complex,[3] is a common component of organic light-emitting diodes (OLEDs). Substituents on the quinoline ring affect the luminescence properties.[4]
In its photo-induced excited-state, 8-hydroxyquinoline converts to zwitterionic isomers, in which the hydrogen atom is transferred from oxygen to nitrogen.[5]
Bioactivity
[編輯]The complexes as well as the heterocycle itself exhibit antiseptic, disinfectant, and pesticide properties,[6][7] functioning as a transcription inhibitor.[8] Its solution in alcohol is used in liquid bandages. It once was of interest as an anti-cancer drug.[9]
A thiol analogue, 8-mercaptoquinoline is also known.[10]
The roots of the invasive plant Centaurea diffusa release 8-hydroxyquinoline, which has a negative effect on plants that have not co-evolved with it.[11]
See also
[編輯]- Clioquinol, an antifungal drug and antiprotozoal drug.
- PBT2
- QUPIC
- Ionophore
- Trace metal detection test
References
[編輯]- ^ Albert, A.; Phillips, J. N. 264. Ionization Constants of Heterocyclic Substances. Part II. Hydroxy-Derivatives of Nitrogenous Six-Membered Ring-Compounds. Journal of the Chemical Society (Resumed). 1956, 1956: 1294–1304. doi:10.1039/JR9560001294.
- ^ Cölle, M.; Dinnebier, R. E.; Brütting, W. The structure of the blue luminescent δ-phase of tris(8-hydroxyquinoline)aluminium(III) (Alq3). Chemical Communications. 2002, 2002 (23): 2908–9. PMID 12478807. S2CID 96135270. doi:10.1039/b209164j.
- ^ Katakura, R.; Koide, Y. Configuration-Specific Synthesis of the Facial and Meridional Isomers of Tris(8-hydroxyquinolinate)aluminum (Alq3). Inorganic Chemistry. 2006, 45 (15): 5730–5732. PMID 16841973. doi:10.1021/ic060594s.
- ^ Montes, V. A.; Pohl, R.; Shinar, J.; Anzenbacher, P. Jr. Effective Manipulation of the Electronic Effects and Its Influence on the Emission of 5-Substituted Tris(8-quinolinolate) Aluminum(III) Complexes. Chemistry: A European Journal. 2006, 12 (17): 4523–4535. PMID 16619313. doi:10.1002/chem.200501403.
- ^ Bardez, E.; Devol, I.; Larrey, B.; Valeur, B. Excited-State Processes in 8-Hydroxyquinoline: Photoinduced Tautomerization and Solvation Effects. The Journal of Physical Chemistry B. 1997, 101 (39): 7786–7793. doi:10.1021/jp971293u.
- ^ Phillips, J. P. The Reactions of 8-Quinolinol. Chemical Reviews. 1956, 56 (2): 271–297. doi:10.1021/cr50008a003.
- ^ 8-Hydroxyquinoline. Medical Dictionary Online. [2016-03-09]. (原始內容存檔於2016-10-09).
- ^ 8-Hydroxyquinoline. Sigma-Aldrich. [2022-02-15].
- ^ Shen, A. Y.; Wu, S. N.; Chiu, C. T. Synthesis and Cytotoxicity Evaluation of some 8-Hydroxyquinoline Derivatives. Journal of Pharmacy and Pharmacology. 1999, 51 (5): 543–548. PMID 10411213. S2CID 33085238. doi:10.1211/0022357991772826
 .
.
- ^ Fleischer, H. Structural Chemistry of Complexes of (n-1)d10ns Metal Ions with β-N-Donor Substituted Thiolate Ligands (m=0, 2). Coordination Chemistry Reviews. 2005, 249 (7–8): 799–827. doi:10.1016/j.ccr.2004.08.024.
- ^ Vivanco, J.M.; Bais, H.P.; Stermitz, F.R.; Thelen, G.C.; Callaway, R.M. Biogeographical variation in community response to root allelochemistry: novel weapons and exotic invasion. Ecology Letters. 2004, 7 (4): 285–292. doi:10.1111/j.1461-0248.2004.00576.x.
