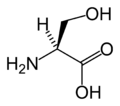
絲胺酸
此條目可參照英語維基百科相應條目來擴充。 |
| 絲胺酸 | |||
|---|---|---|---|
| |||

| |||
| IUPAC名 Serine | |||
| 別名 | 2-Amino-3-hydroxypropanoic acid | ||
| 識別 | |||
| CAS編號 | 56-45-1 ? 302-84-1 312-84-5((D-isomer)) | ||
| PubChem | 617 | ||
| ChemSpider | 5736 (L-form), 597 | ||
| SMILES |
| ||
| InChI |
| ||
| EC編號 | 206-130-6 | ||
| ChEBI | 17115 | ||
| DrugBank | DB00133 | ||
| IUPHAR配位基 | 726 | ||
| 性質[2] | |||
| 化學式 | C3H7NO3 | ||
| 莫耳質量 | 105.09 g·mol−1 | ||
| 外觀 | 白色晶體或粉末 | ||
| 密度 | 1.603 g/cm3 (22 °C) | ||
| 熔點 | 246 °C 分解 | ||
| 溶解性(水) | 可溶 | ||
| pKa | 2.21 (carboxyl), 9.15 (amino)[1] | ||
| 若非註明,所有資料均出自標準狀態(25 ℃,100 kPa)下。 | |||
絲胺酸(英語:serine,三字代碼:Ser,一字代碼:S)是一種非必需的基本胺基酸,中心碳原子帶有羥甲基側鏈,為極性胺基酸。其密碼子為UCU、UCC、UCA、UCG、AGU與AGC。絲胺酸富含於雞蛋、魚和黃豆。絲胺酸可促進脂肪和脂肪酸的新陳代謝,有助於維持免疫系統。其在醫藥上有著廣泛用途。
發現
[編輯]
絲胺酸是基本胺基酸之一,自然狀態下僅以左旋形式存在於蛋白質中。絲胺酸為非必需胺基酸,其在人體內可通過甘胺酸合成。絲胺酸最先於1865年由德國化學家埃米爾·克萊默從富含該成分的絲蛋白中提取[3],得名自拉丁文中的絲綢,sericum。其化學結構於1902年被測定。[4][5]
合成與化學反應
[編輯]工業上以甘胺酸和甲醇為原料,由羥甲基轉移酶催化合成L-絲胺酸[6]。
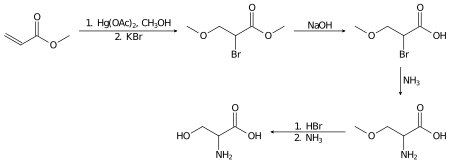
消旋絲胺酸則可以在實驗室中,以丙烯酸甲酯為原料,通過以下幾步製備[7]:
絲胺酸的加氫反應則生成一種二醇,絲氨醇:
- HOCH
2CH(NH
2)CO
2H + 2 H
2 → HOCH
2CH(NH
2)CH
2OH + 2 H
2O
生物功能
[編輯]新陳代謝
[編輯]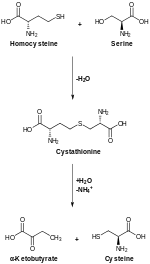
絲胺酸參與多種物質的生物合成,因而在新陳代謝中起重要作用。絲胺酸參與嘌呤與嘧啶的合成,亦是合成多種胺基酸的前體,包括甘胺酸與半胱胺酸,以及細菌合成途徑下產生的酪胺酸。絲胺酸亦是鞘脂與葉酸的前體,這兩種物質是體內生物合成重要的一碳片段供體。[來源請求]
傳訊分子
[編輯]D-絲胺酸,作為一種神經調質,可在神經元中由L -絲胺酸經絲胺酸消旋酶催化合成,主要作為NMDA受體上甘胺酸位點(NR1)的強力興奮劑,使受體在結合麩胺酸活化後得以開放離子通道。對於NMDA受體離子通道的開放,麩胺酸以及甘胺酸(或D-絲胺酸)的結合是必不可少的,除此之外不能有通道阻斷劑作用(如Mg2+或Zn2+ )[8]。實際上,D-絲胺酸對於甘胺酸位點的作用比甘胺酸自身更強。[9][10]D-絲胺酸曾被認為只存在於細菌中,直到最近的研究表明其為第二種自然存在於人體內的D 胺基酸(另一種為不久前發現的D- 天門冬胺酸),NMDA受體上的甘胺酸位點也可能因此更名D- 絲胺酸位點[11] 。除中樞神經系統外,D -絲胺酸亦可在外周組織發揮傳訊分子作用,如在軟骨[12] 、腎臟[13] 以及陰莖海綿體[14]。
參考文獻
[編輯]- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Weast, Robert C. (編). CRC Handbook of Chemistry and Physics 62nd. Boca Raton, FL: CRC Press. 1981: C-512. ISBN 0-8493-0462-8..
- ^ Cramer, Emil. Ueber die Bestandtheile der Seide [On the constituents of silk]. Journal für praktische Chemie. 1865, 96: 76–98 [2023-09-16]. (原始內容存檔於2022-11-06) (German). Serine is named on p. 93: "Ich werde den in Frage stehenden Körper unter dem Namen Serin beschreiben." (I will describe the body [i.e., substance] in question by the name "serine".)
- ^ Fischer, Emil; Leuchs, Hermann. Synthese des Serins, der l-Glucosaminsäure und anderer Oxyaminosäuren [Synthesis of serine, of l-glucosaminic acid, and other oxyamino acids]. Berichte der Deutschen Chemischen Gesellschaft. 1902, 35 (3): 3787–3805. doi:10.1002/cber.190203503213 (德語).
- ^ Serine. The Columbia Encyclopedia 6th ed.. encyclopedia.com. [22 October 2012]. (原始內容存檔於2016-08-10).
- ^ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker, Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, 2005, doi:10.1002/14356007.a02_057.pub2
- ^ Carter HE, West HD. dl-Serine. Org. Synth. 1940, 20: 81 [2023-09-19]. doi:10.15227/orgsyn.020.0081. (原始內容存檔於2023-01-30).
- ^ Liu Y, Hill RH, Arhem P, von Euler G. NMDA and glycine regulate the affinity of the Mg2+-block site in NR1-1a/NR2A NMDA receptor channels expressed in Xenopus oocytes. Life Sciences. 2001, 68 (16): 1817–1826. PMID 11292060. doi:10.1016/S0024-3205(01)00975-4.
- ^ MacKay, Mary-Anne B.; Kravtsenyuk, Maryana; Thomas, Rejish; Mitchell, Nicholas D.; Dursun, Serdar M.; Baker, Glen B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression?. Frontiers in Psychiatry. 6 February 2019, 10: 25. ISSN 1664-0640. PMC 6372501
 . PMID 30787885. doi:10.3389/fpsyt.2019.00025
. PMID 30787885. doi:10.3389/fpsyt.2019.00025  .
. D-Serine is more potent than glycine as a coagonist at the NMDA receptor, has a regional distribution in the brain that is similar to that of NMDA receptors and appears to be more closely associated with synaptic NMDA receptors than glycine (which is more closely associated with non-synaptic NMDA receptors).
- ^ Wolosker, Herman; Balu, Darrick T. D-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders. Translational Psychiatry. 9 June 2020, 10 (1): 184. ISSN 2158-3188. PMC 7283225
 . PMID 32518273. doi:10.1038/s41398-020-00870-x.
. PMID 32518273. doi:10.1038/s41398-020-00870-x. D-Serine is functionally a more potent activator of synaptic NMDARs than glycine, and mounting evidence suggests that it serves as the major NMDAR co-agonist in limbic brain regions implicated in neuropsychiatric disorders.
- ^ Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. Apr 2000, 97 (9): 4926–4931. Bibcode:2000PNAS...97.4926M. PMC 18334
 . PMID 10781100. doi:10.1073/pnas.97.9.4926
. PMID 10781100. doi:10.1073/pnas.97.9.4926  .
.
- ^ Takarada T, Hinoi E, Takahata Y, Yoneda Y. Serine racemase suppresses chondrogenic differentiation in cartilage in a Sox9-dependent manner. Journal of Cellular Physiology. May 2008, 215 (2): 320–328. PMID 17929246. S2CID 45669104. doi:10.1002/jcp.21310.
- ^ Ma MC, Huang HS, Chen YS, Lee SH. Mechanosensitive N-methyl-D-aspartate receptors contribute to sensory activation in the rat renal pelvis. Hypertension. Nov 2008, 52 (5): 938–944. PMID 18809793. doi:10.1161/HYPERTENSIONAHA.108.114116
 .
.
- ^ Ghasemi M, Rezania F, Lewin J, Moore KP, Mani AR. D-Serine modulates neurogenic relaxation in rat corpus cavernosum. Biochemical Pharmacology. Jun 2010, 79 (12): 1791–1796. PMID 20170643. doi:10.1016/j.bcp.2010.02.007.