奥昔布宁
外观
 | |
 | |
| 临床资料 | |
|---|---|
| 商品名 | Ditropan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682141 |
| 核准状况 | |
| 怀孕分级 |
|
| 给药途径 | 口服给药、transdermal gel、transdermal patch |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 血浆蛋白结合率 | 91–93% |
| 生物半衰期 | 12.4–13.2 hours |
| 识别信息 | |
| |
| CAS号 | 5633-20-5 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.590 |
| 化学信息 | |
| 化学式 | C22H31NO3 |
| 摩尔质量 | 357.486 g/mol |
| 3D模型(JSmol) | |
| |
| |
奥昔布宁(Oxybutynin),商品名Ditropan、Lyrinel XL、Lenditro (ZA)、Driptane (RU)、Uripan (Middle East)[1],为一种抗胆碱剂,可以缓解膀胱排尿困难,包含频尿及尿失禁等症状,可以降低膀胱肌肉痉挛[2]。本品也可用于帮助缓解与肾结石相关的症状。
本品可竞争性拮抗M1、M2,及M3等蕈毒碱型乙酰胆碱受体。
立体化学
[编辑]奥昔布宁含有一个立体中心,由两种对映体组成。这是外消旋体,即1:(R)的1:1混合物 - 和(S)形式:[6][7]
| 奥昔布宁的对映体 | |
|---|---|
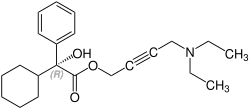 CAS-Nummer: 119618-21-2 |
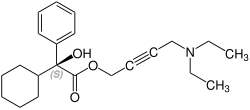 CAS-Nummer: 119618-22-3 |
参考文献
[编辑]- ^ Uripan Tablets. Adwia Pharmaceuticals. [22 November 2015]. (原始内容存档于2020-10-26).
- ^ Chapple CR. "Muscarinic receptor antagonists in the treatment of overactive bladder". Urology (55)5, Supp. 1:33-46, 2000.
- ^ Tupker RA, Harmsze AM, Deneer VH. Oxybutynin therapy for generalized hyperhidrosis.. Arch Dermatol. 2006, 142 (8): 1065–6. PMID 16924061. doi:10.1001/archderm.142.8.1065.
- ^ Mijnhout GS, Kloosterman H, Simsek S, Strack van Schijndel RJ, Netelenbos JC. Oxybutynin: dry days for patients with hyperhidrosis.. Neth J Med. 2006, 64 (9): 326–8. PMID 17057269.
- ^ Schollhammer M, Misery L. Treatment of hyperhidrosis with oxybutynin.. Arch Dermatol. 2007, 143 (4): 544–5. PMID 17438194. doi:10.1001/archderm.143.4.544.
- ^ Kachur JF, et al. R and S enantiomers of oxybutynin: pharmacological effects in guinea pig bladder and intestine, Journal of Pharmacology and Experimental Therapeutics, 247, S. 867–872, 1988; PMID 2849672.
- ^ Noronha-Blob L, Kachur JF. Enantiomers of oxybutynin: in vitro pharmacological characterization at M1, M2 and M3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs, Journal of Pharmacology and Experimental Therapeutics, 256, S. 562–567, 1991; PMID 1993995.
