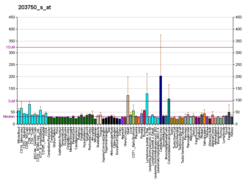
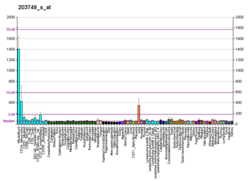
视黄酸受体α
外观
视黄酸受体α(英語:Retinoic acid receptor alpha,RAR-α),也称为NR1B1(核受体第一亚族B组成员1,nuclear receptor subfamily 1, group B, member 1)是一个核受体,在人类基因组中由 RARA 基因编码。[6][7]
相互作用
[编辑]视黄酸受体α可以与下列蛋白質发生进行交互作用:
参考文献
[编辑]- ^ 對retinoic acid receptor alpha起作用的藥物;在維基數據上查看/編輯參考.
- ^ 2.0 2.1 2.2 GRCh38: Ensembl release 89: ENSG00000131759 - Ensembl, May 2017
- ^ 3.0 3.1 3.2 GRCm38: Ensembl release 89: ENSMUSG00000037992 - Ensembl, May 2017
- ^ Human PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mouse PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Identification of a receptor for the morphogen retinoic acid. Nature. 1987, 330 (6149): 624–9. PMID 2825036. doi:10.1038/330624a0.
- ^ High-density genetic map of the BRCA1 region of chromosome 17q12-q21. Genomics. September 1993, 17 (3): 618–23. PMID 8244378. doi:10.1006/geno.1993.1381.
- ^ Liu R, Takayama S, Zheng Y, Froesch B, Chen GQ, Zhang X, Reed JC, Zhang XK. Interaction of BAG-1 with retinoic acid receptor and its inhibition of retinoic acid-induced apoptosis in cancer cells. J. Biol. Chem. July 1998, 273 (27): 16985–92. PMID 9642262. doi:10.1074/jbc.273.27.16985.
- ^ 9.0 9.1 McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. June 2001, 105 (7): 877–89. PMID 11439184. doi:10.1016/S0092-8674(01)00401-9.
- ^ Despouy G, Bastie JN, Deshaies S, Balitrand N, Mazharian A, Rochette-Egly C, Chomienne C, Delva L. Cyclin D3 is a cofactor of retinoic acid receptors, modulating their activity in the presence of cellular retinoic acid-binding protein II. J. Biol. Chem. February 2003, 278 (8): 6355–62. PMID 12482873. doi:10.1074/jbc.M210697200.
- ^ Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW. Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol. Endocrinol. February 2001, 15 (2): 241–54. PMID 11158331. doi:10.1210/me.15.2.241.
- ^ Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J. Biol. Chem. November 1999, 274 (48): 34283–93. PMID 10567404. doi:10.1074/jbc.274.48.34283.
- ^ Ko L, Cardona GR, Chin WW. Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc. Natl. Acad. Sci. U.S.A. May 2000, 97 (11): 6212–7. PMC 18584
 . PMID 10823961. doi:10.1073/pnas.97.11.6212.
. PMID 10823961. doi:10.1073/pnas.97.11.6212.
- ^ Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. Identification of nuclear receptor corepressor as a peroxisome proliferator-activated receptor alpha interacting protein. J. Biol. Chem. May 1999, 274 (22): 15901–7. PMID 10336495. doi:10.1074/jbc.274.22.15901.
- ^ Guidez F, Ivins S, Zhu J, Söderström M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. April 1998, 91 (8): 2634–42. PMID 9531570.
- ^ Dong S, Tweardy DJ. Interactions of STAT5b-RARalpha, a novel acute promyelocytic leukemia fusion protein, with retinoic acid receptor and STAT3 signaling pathways. Blood. April 2002, 99 (8): 2637–46. PMID 11929748. doi:10.1182/blood.V99.8.2637.
- ^ Hong SH, David G, Wong CW, Dejean A, Privalsky ML. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARalpha) and PLZF-RARalpha oncoproteins associated with acute promyelocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. August 1997, 94 (17): 9028–33. PMC 23013
 . PMID 9256429. doi:10.1073/pnas.94.17.9028.
. PMID 9256429. doi:10.1073/pnas.94.17.9028.
- ^ Hu X, Chen Y, Farooqui M, Thomas MC, Chiang CM, Wei LN. Suppressive effect of receptor-interacting protein 140 on coregulator binding to retinoic acid receptor complexes, histone-modifying enzyme activity, and gene activation. J. Biol. Chem. January 2004, 279 (1): 319–25. PMID 14581481. doi:10.1074/jbc.M307621200.
- ^ Farooqui M, Franco PJ, Thompson J, Kagechika H, Chandraratna RA, Banaszak L, Wei LN. Effects of retinoid ligands on RIP140: molecular interaction with retinoid receptors and biological activity. Biochemistry. February 2003, 42 (4): 971–9. PMID 12549917. doi:10.1021/bi020497k.
- ^ L'Horset F, Dauvois S, Heery DM, Cavaillès V, Parker MG. RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol. Cell. Biol. November 1996, 16 (11): 6029–36. PMC 231605
 . PMID 8887632.
. PMID 8887632.
- ^ Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. May 1996, 272 (5266): 1336–9. PMID 8650544. doi:10.1126/science.272.5266.1336.
- ^ Seol W, Hanstein B, Brown M, Moore DD. Inhibition of estrogen receptor action by the orphan receptor SHP (short heterodimer partner). Mol. Endocrinol. October 1998, 12 (10): 1551–7. PMID 9773978. doi:10.1210/me.12.10.1551.
- ^ Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. April 1995, 9 (7): 769–82. PMID 7705655. doi:10.1101/gad.9.7.769.
- ^ Zhong S, Delva L, Rachez C, Cenciarelli C, Gandini D, Zhang H, Kalantry S, Freedman LP, Pandolfi PP. A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARalpha and T18 oncoproteins. Nat. Genet. November 1999, 23 (3): 287–95. PMID 10610177. doi:10.1038/15463.
- ^ Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol. Cell. Biol. July 2002, 22 (13): 4522–34. PMC 133906
 . PMID 12052862. doi:10.1128/MCB.22.13.4522-4534.2002.
. PMID 12052862. doi:10.1128/MCB.22.13.4522-4534.2002.
- ^ Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. April 1992, 11 (4): 1409–18. PMC 556590
 . PMID 1314167.
. PMID 1314167.
- ^ Kim HJ, Yi JY, Sung HS, Moore DD, Jhun BH, Lee YC, Lee JW. Activating signal cointegrator 1, a novel transcription coactivator of nuclear receptors, and its cytosolic localization under conditions of serum deprivation. Mol. Cell. Biol. September 1999, 19 (9): 6323–32. PMC 84603
 . PMID 10454579. doi:10.1128/mcb.19.9.6323.
. PMID 10454579. doi:10.1128/mcb.19.9.6323.
- ^ He B, Wilson EM. Electrostatic modulation in steroid receptor recruitment of LXXLL and FXXLF motifs. Mol. Cell. Biol. March 2003, 23 (6): 2135–50. PMC 149467
 . PMID 12612084. doi:10.1128/MCB.23.6.2135-2150.2003.
. PMID 12612084. doi:10.1128/MCB.23.6.2135-2150.2003.
- ^ Zeng M, Kumar A, Meng G, Gao Q, Dimri G, Wazer D, Band H, Band V. Human papilloma virus 16 E6 oncoprotein inhibits retinoic X receptor-mediated transactivation by targeting human ADA3 coactivator. J. Biol. Chem. November 2002, 277 (47): 45611–8. PMID 12235159. doi:10.1074/jbc.M208447200.
- ^ Martin PJ, Delmotte MH, Formstecher P, Lefebvre P. PLZF is a negative regulator of retinoic acid receptor transcriptional activity. Nucl. Recept. September 2003, 1 (1): 6. PMC 212040
 . PMID 14521715. doi:10.1186/1478-1336-1-6.
. PMID 14521715. doi:10.1186/1478-1336-1-6.
延伸阅读
[编辑]- Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1988, 330 (6147): 444–50. PMID 2825025. doi:10.1038/330444a0.
- Sirulnik A, Melnick A, Zelent A, Licht JD. Molecular pathogenesis of acute promyelocytic leukaemia and APL variants. Best Pract Res Clin Haematol. 2003, 16 (3): 387–408. PMID 12935958. doi:10.1016/S1521-6926(03)00062-8.
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992, 355 (6359): 446–9. PMID 1310351. doi:10.1038/355446a0.
- Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub MP, Durand B, Lanotte M, Berger R, Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992, 11 (2): 629–42. PMC 556495
 . PMID 1311253.
. PMID 1311253. - Baniahmad A, Köhne AC, Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992, 11 (3): 1015–23. PMC 556542
 . PMID 1347744.
. PMID 1347744. - de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991, 66 (4): 675–84. PMID 1652369. doi:10.1016/0092-8674(91)90113-D.
- de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990, 347 (6293): 558–61. PMID 2170850. doi:10.1038/347558a0.
- Brand NJ, Petkovich M, Chambon P. Characterization of a functional promoter for the human retinoic acid receptor-alpha (hRAR-alpha). Nucleic Acids Res. 1990, 18 (23): 6799–806. PMC 332734
 . PMID 2175878. doi:10.1093/nar/18.23.6799.
. PMID 2175878. doi:10.1093/nar/18.23.6799. - Borrow J, Goddard AD, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990, 249 (4976): 1577–80. PMID 2218500. doi:10.1126/science.2218500.
- Arveiler B, Petkovich M, Mandel JL, Chambon P. A PstI RFLP for the human retinoic acid receptor in 17q21. Nucleic Acids Res. 1988, 16 (13): 6252. PMC 336887
 . PMID 2899875. doi:10.1093/nar/16.13.6252.
. PMID 2899875. doi:10.1093/nar/16.13.6252. - Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995, 377 (6548): 454–7. PMID 7566127. doi:10.1038/377454a0.
- Fisher GJ, Talwar HS, Xiao JH, Datta SC, Reddy AP, Gaub MP, Rochette-Egly C, Chambon P, Voorhees JJ. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J. Biol. Chem. 1994, 269 (32): 20629–35. PMID 8051161.
- Chen Z, Guidez F, Rousselot P, Agadir A, Chen SJ, Wang ZY, Degos L, Zelent A, Waxman S, Chomienne C. PLZF-RAR alpha fusion proteins generated from the variant t(11;17)(q23;q21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid receptors. Proc. Natl. Acad. Sci. U.S.A. 1994, 91 (3): 1178–82. PMC 521477
 . PMID 8302850. doi:10.1073/pnas.91.3.1178.
. PMID 8302850. doi:10.1073/pnas.91.3.1178. - Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996, 87 (3): 882–6. PMID 8562957.
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996, 85 (3): 403–14. PMID 8616895. doi:10.1016/S0092-8674(00)81118-6.
- Liu W, Hellman P, Li Q, Yu WR, Juhlin C, Nordlinder H, Rollman O, Akerström G, Törmä H, Melhus H. Biosynthesis and function of all-trans- and 9-cis-retinoic acid in parathyroid cells. Biochem. Biophys. Res. Commun. 1996, 229 (3): 922–9. PMID 9005841. doi:10.1006/bbrc.1996.1903.
- Thénot S, Henriquet C, Rochefort H, Cavaillès V. Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1. J. Biol. Chem. 1997, 272 (18): 12062–8. PMID 9115274. doi:10.1074/jbc.272.18.12062.