乙酰克里定
外观
 | |
 | |
| 臨床資料 | |
|---|---|
| AHFS/Drugs.com | 国际药品名称 |
| 给药途径 | Topical (ophthalmic solution) |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 药物代谢 | deacetylation? |
| 识别信息 | |
| |
| CAS号 | 827-61-2 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.431 |
| 化学信息 | |
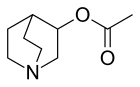
| 化学式 | C9H15NO2 |
| 摩尔质量 | 169.22 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
乙酰克里定或醋克利定是一种有机化合物,化学式为C9H15NO2。它可由3-喹核醇和乙酸酐反应制得。[1]它和3-溴溴苄反应,得到季铵盐N-(3-溴苄基)乙酰克里定溴化物。[2]
参考文献
[编辑]- ^ M. H. Shaw, V. W. Shurtleff, J. A. Terrett, J. D. Cuthbertson, D. W. C. MacMillan. Native functionality in triple catalytic cross-coupling: sp3 C-H bonds as latent nucleophiles. Science. 2016-06-10, 352 (6291): 1304–1308 [2021-08-15]. ISSN 0036-8075. PMC 5114852
 . PMID 27127237. doi:10.1126/science.aaf6635 (英语).
. PMID 27127237. doi:10.1126/science.aaf6635 (英语).
- ^ Primozic, Ines; Bolant, Marijana; Ramic, Alma; Tomic, Srdanka. Preparation of novel meta and para substituted N-benzyl protected quinuclidine esters and their resolution with butyrylcholinesterase. International Electronic Conference on Synthetic Organic Chemistry, 15th, Nov. 1-30, 2011. ISBN 3-906980-25-1.
